Reputable Manufacturer Supply 330784-47-9 with Fast Shipping, Buy Quality Avanafil
- Molecular Formula:C23H26ClN7O3
- Molecular Weight:483.958
- Appearance/Colour:White solid
- Melting Point:150-152 °C
- Refractive Index:1.651
- PKA:11.84±0.46(Predicted)
- PSA:125.39000
- Density:1.373 g/cm3
- LogP:2.96070
Avanafil(Cas 330784-47-9) Usage
|
Description
|
Avanafil is a PDE5 inhibitor approved for erectile dysfunction by the FDA on April 27, 2012 and by EMA on June 21, 2013. Avanafil is sold under the brand names Stendra and Spedra. |
|
Chemical Properties
|
White Solid
|
|
Originator
|
Mitsubishi Tanabe Pharma Corporation (Japan)
|
|
Uses
|
Avanafil is used to treat men who have erectile dysfunction (also called sexual impotence). Avanafil belongs to a group of medicines called phosphodiesterase 5 (PDE5) inhibitors. These medicines prevent an enzyme called phosphodiesterase type-5 from working too quickly. Clinical studies of avanafil (the active ingredient in Stendra) and sildenafil (the active ingredient in Viagra) show that both medications are very effective at treating erectile dysfunction for most men. Despite being a relatively new medication, Stendra is backed up by several large-scale studies of men with ED. |
|
Brand name
|
Zepeed
|
InChI:InChI=1/C23H26ClN7O3/c1-34-19-6-5-15(10-18(19)24)11-27-21-17(22(33)28-13-20-25-7-3-8-26-20)12-29-23(30-21)31-9-2-4-16(31)14-32/h3,5-8,10,12,16,32H,2,4,9,11,13-14H2,1H3,(H,28,33)(H,27,29,30)/t16-/m0/s1
330784-47-9 Relevant articles
Method of preparing medical compound stendra
-
Paragraph 0014; 0037-0038; 0048-0049; 0059-0060, (2019/02/04)
The invention discloses a method of prep...
Preparation method of Stendra
-
Paragraph 0032-0058, (2019/06/07)
The invention provides a preparation met...
A pharmaceutical compound atorvastatin that non-synthetic method (by machine translation)
-
Paragraph 0012; 0028; 0029; 0034; 0035; 0042, (2019/02/10)
The present invention discloses a pharma...
Preparation method of pharmaceutical compound avanafil
-
Paragraph 0014; 0027; 0036; 0037; 0049; 0060, (2019/02/10)
The invention discloses a preparation me...
330784-47-9 Process route
-
-
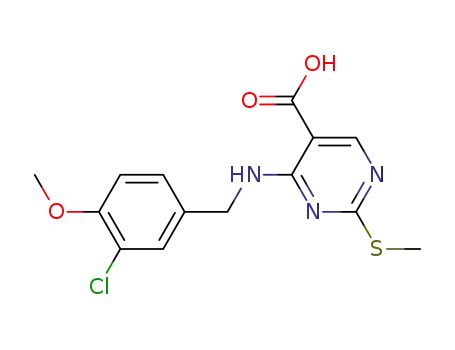
4-[(3-chloro-4-methoxybenzyl)amino]-2-methanesulfonyl-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide
-
- 23356-96-9
(S)-1-Pyrrolidin-2-yl-methanol
Conditions
| Conditions |
Yield |
|
With N-ethyl-N,N-diisopropylamine; In chloroform; at 50 ℃; Temperature;
|
90.5% |
|
With N-ethyl-N,N-diisopropylamine; In isopropyl alcohol; at 20 ℃; for 5h;
|
87.5% |
|
With N-ethyl-N,N-diisopropylamine; In dichloromethane; at 0 ℃; Temperature; Green chemistry;
|
87% |
|
4-[(3-chloro-4-methoxybenzyl)amino]-2-methanesulfonyl-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide; With N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 0 ℃; for 0.25h;
(S)-1-Pyrrolidin-2-yl-methanol; In N,N-dimethyl-formamide; at 20 ℃; for 4h; Reagent/catalyst; Solvent; Temperature;
|
74% |
|
at 20 - 120 ℃;
|
|
|
With triethylamine; In ethyl acetate; at 20 - 30 ℃;
|
0.7 g |
-
- 330785-84-7
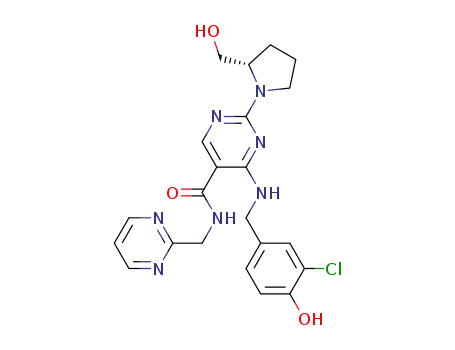
4-{[(3-chloro-4-methoxyphenyl)methyl]amino}-2-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]pyrimidine-5-carboxylic acid
-
- 75985-45-4
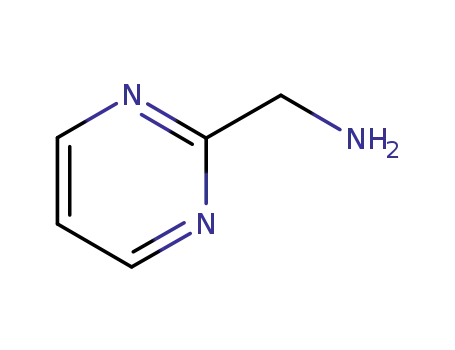
2-aminomethylpyrimidine
Conditions
| Conditions |
Yield |
|
With dicyclohexyl-carbodiimide; 1-hydroxy-1,2,3-benzotriazine-4(3H)-one; In N,N-dimethyl-formamide; at 0 ℃; Temperature;
|
99% |
|
With 5, 10, 15, 20-tetrakis[4-(dihydroxyboryl)phenyl]-21H,23H-porphine; In toluene; for 16h; Time; Reflux; Green chemistry;
|
91.2% |
|
With benzotriazol-1-ol; dicyclohexyl-carbodiimide; In dimethyl sulfoxide; at 20 ℃; for 4h;
|
90% |
|
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In DMF (N,N-dimethyl-formamide); at 20 ℃; for 8h;
|
|
330784-47-9 Upstream products
-
330785-84-7
4-{[(3-chloro-4-methoxyphenyl)methyl]amino}-2-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]pyrimidine-5-carboxylic acid
-
75985-45-4
2-aminomethylpyrimidine
-
330786-34-0
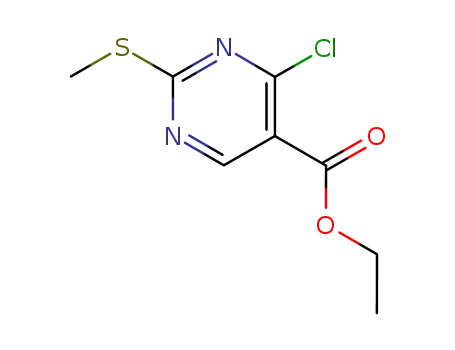
4-[(3-chloro-4-methoxybenzyl)amino]-2-(methylsulfanyl)pyrimidine-5-carboxylic acid
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester
330784-47-9 Downstream products
 English
English English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego

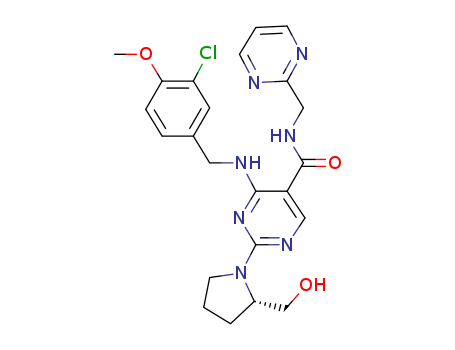
![4-[(3-chloro-4-methoxybenzyl)amino]-2-methanesulfonyl-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide](/upload/2024/4/be7621a3-d119-4d22-8f42-fe78d1867e09.png)

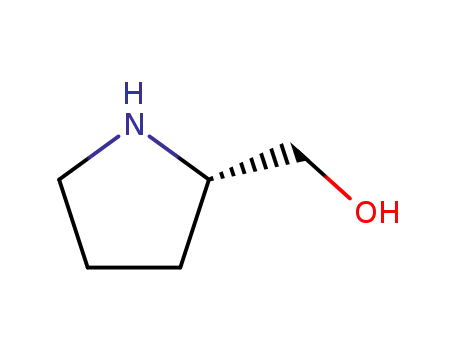

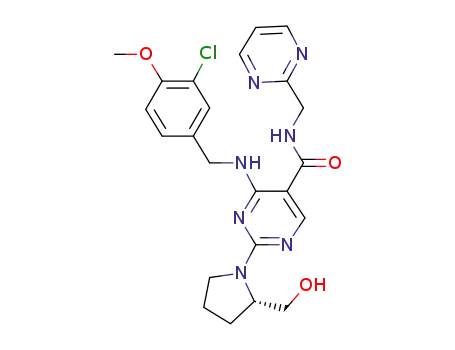
![4-{[(3-chloro-4-methoxyphenyl)methyl]amino}-2-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]pyrimidine-5-carboxylic acid](/upload/2024/4/a9255e55-5f54-4da9-bd6c-c40bb81f2181.png)