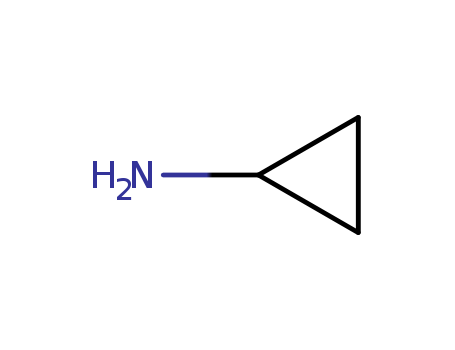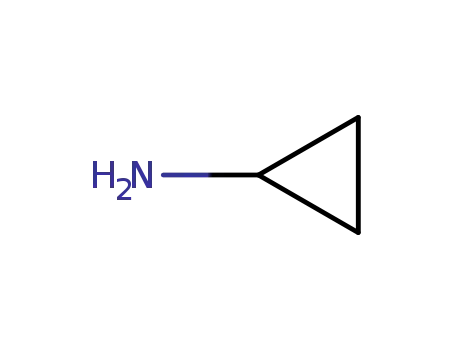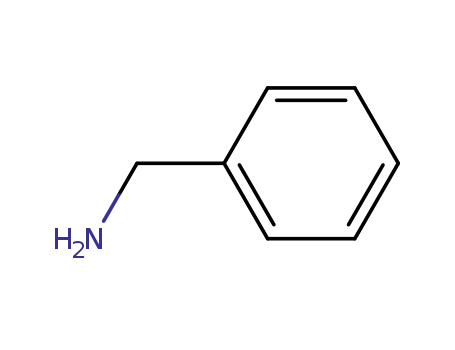Trustworthy Factory Supply 765-30-0 with Safe Shipping, Buy Quality Cyclopropylamine
- Molecular Formula:C3H7N
- Molecular Weight:57.0953
- Appearance/Colour:Clear and colourless, volatile liquid
- Vapor Pressure:4.67 psi ( 20 °C)
- Melting Point:-50 °C
- Refractive Index:1.4206
- Boiling Point:49.3 °C at 760 mmHg
- PKA:pK1:9.10(+1) (25°C)
- Flash Point:-14 °F
- PSA:26.02000
- Density:0.938 g/cm3
- LogP:0.80780
Cyclopropylamine(Cas 765-30-0) Usage
|
Chemical Properties
|
Cyclopropylamine (CPA) is a primary aliphatic amine with the formula C3H7N. It's a mouse metabolite and an essential intermediate in the preparation of many biologically active substances. It is a colorless and transparent flammable liquid with volatility and ammonia odor. It is miscible with water, methanol, ethanol, benzene, toluene and other solvents.
|
|
Uses
|
Cyclopropylamine is mainly used in organic synthesis and pharmaceutical synthesis intermediates. It can be used in the synthesis of new antibacterial drugs such as ciprofloxacin, enrofloxacin, and spafloxacin. Cyclopropylamine is a primary aliphatic amine that consists of cyclopropane bearing a single amino substituent. It has a role as a mouse metabolite. |
|
Biochem/physiol Actions
|
Cyclopropylamine inactivates cytochrome P450 enzymes by a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. It is a mechanism-based inhibitor of quinoprotein methylamine dehydrogenase from Paracoccus denitrificans.
|
|
Purification Methods
|
It has been isolated as the benzamide m 100.6-101.0o (from aqueous EtOH). It forms a picrate m 149o (from EtOH/pet ether) from which the free base can be recovered using a basic ion-exchange resin and can then be distilled through a Todd column (p 11) using an automatic still head which only collects products boiling below 51o/atm. Polymeric materials if present will boil above this temperature. The hydrochloride has m 85-86o [Roberts & Chambers J Am Chem Soc 73 5030 1951, Jones J Org Chem 9 484 1944, Emmons J Am Chem Soc 79 6522 1957]. [Beilstein 12 IV 3.]
|
InChI:InChI=1/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
765-30-0 Relevant articles
The Insertion Reaction of NH Singlet Radicals into the C-H Bonds of Cyclopropane and Isobutane in the Liquid Phase
Hamada, Jun-ich,Tsunashima, Shigeru,Sato, Shin
, p. 1739 - 1742 (1982)
Hydrogen azide was photolyzed in liquid ...
Method for preparing amine through catalytic reduction of nitro compound by cyclic (alkyl) (amino) carbene chromium complex
-
Paragraph 0015, (2021/04/17)
The cyclic (alkyl) (amino) carbene chrom...
Novel method and technology for synthesizing cyclopropyl ammonia
-
Paragraph 0019, (2021/09/21)
The invention discloses a synthesis proc...
O -Phthalaldehyde catalyzed hydrolysis of organophosphinic amides and other P(O)-NH containing compounds
Li, Bin-Jie,Simard, Ryan D.,Beauchemin, André M.
supporting information, p. 8667 - 8670 (2017/08/10)
Over 50 years ago, Jencks and Gilchrist ...
NHC-based coordination polymers as solid molecular catalysts for reductive amination of biomass levulinic acid
Sun, Zheming,Chen, Jiangbo,Tu, Tao
, p. 789 - 794 (2017/08/18)
A class of robust solid molecular NHC-ba...
765-30-0 Process route
-
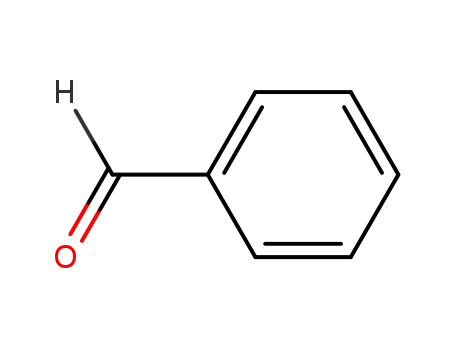
- 13324-66-8
N-benzylcyclopropanamine
-
- 765-30-0
Cyclopropylamine
Conditions
| Conditions |
Yield |
|
With mitochondrial monoamine oxidase; In water; Mechanism; Product distribution; experiments with 14C- and 3H-labelled compounds; comparison of enzyme and electrochemical amine oxidation mechanism (two electron transfers via a radical cation intermediate);
|
|
-
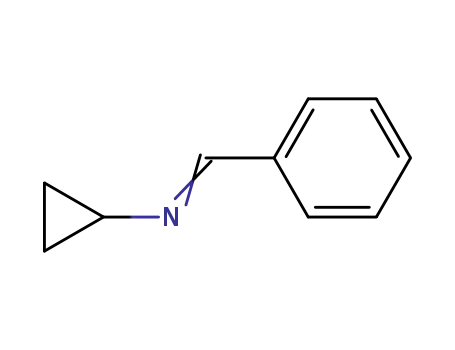
- 3187-77-7
N-benzylidenecyclopropylamine
-
- 765-30-0
Cyclopropylamine
Conditions
| Conditions |
Yield |
|
With 20 μM Tris pH 9.0 buffer; at 25 ℃; Rate constant;
|
|
765-30-0 Upstream products
765-30-0 Downstream products
-
1453-50-5
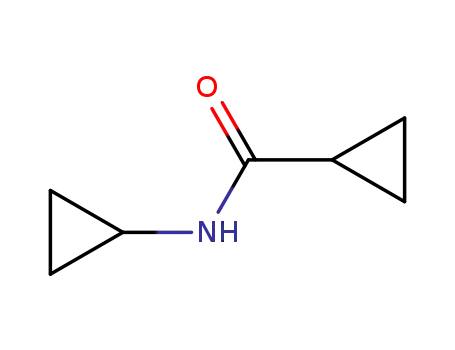
N-cyclopropylcyclopropanecarboxamide
-
15205-35-3
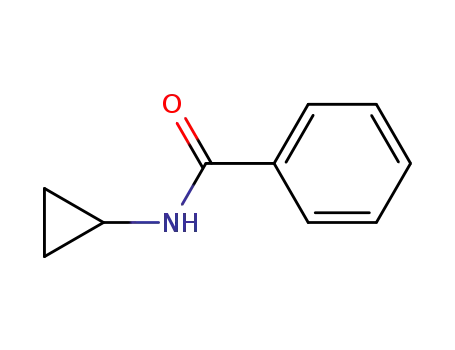
N-cyclopropylbenzamide
-
13140-86-8
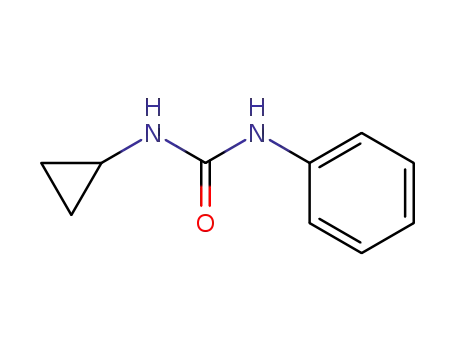
1-cyclopropyl-3-phenylurea
-
77991-97-0
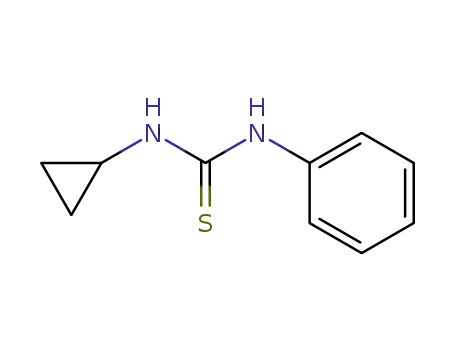
3-cyclopropyl-1-phenylthiourea
 English
English English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego